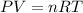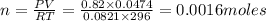Chemistry, 27.11.2019 21:31 robert7248
In an electrolysis experiment such as the one you are going to perform, 47.4 ml of hydrogen gas were collected. the temperature was 23 degrees celsius and the barometric pressure was 643 mm hg. the vapor pressure of water at 23 degrees celsius is 21 mm hg. the number of moles of hydrogen gas formed in the experiment was

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 01:30
Pls! plant cells and animal cells were observed under a microscope. the characteristics of two cells are listed below. cell p: does not capture sunlight cell q: has cytoplasm but no chloroplast which statement about the two cells is correct? cell q also has a cell wall. cell q also has large vacuole. cell p also has a large vacuole. cell p also has a cell membrane.
Answers: 1

Chemistry, 22.06.2019 05:50
What are transitions between a liquid and gas called? identify which way they are transitioning
Answers: 2

Chemistry, 22.06.2019 15:20
Select the most likely product for this reaction: koh(aq) + co2(g) – ? k2co3(aq) + h2o(1) k(s) + h2(g) + o2(g) k(s) + co3(9) +h2
Answers: 2

You know the right answer?
In an electrolysis experiment such as the one you are going to perform, 47.4 ml of hydrogen gas were...
Questions

Social Studies, 23.10.2019 07:00

Mathematics, 23.10.2019 07:00

Arts, 23.10.2019 07:00

Mathematics, 23.10.2019 07:00



History, 23.10.2019 07:00

Chemistry, 23.10.2019 07:00

Biology, 23.10.2019 07:00





Mathematics, 23.10.2019 07:00


Mathematics, 23.10.2019 07:00




History, 23.10.2019 07:00