
Chemistry, 27.11.2019 21:31 jdanstudy9528
1) the equilibrium constant (keq) for the following reaction is 6.2x10-6. for the following assume a temperature of 25 °c. l-malate + nad+«oxaloacetate + nadh + h+inthe cell the dg for the reaction is –10 kj/mol and the ratio of [nadh]/[nad] is 0.1. what must be the ratio of [oxaloacetate] to [l-malate]?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 06:10
Explain the relationship between forward and backward reactions in equilibrium, and predict how changing the amount of a reactant (creating a tension) will affect that relationship.
Answers: 1

Chemistry, 22.06.2019 06:30
Three cards with holes are arranged in a straight line. a light is shined through the first card’s hole and travels through all three cards. what does this tell you about light rays? a) that light is reflected b) that light is refractive c) that light travels in a straight line d) that light does not travel in a straight line
Answers: 1

Chemistry, 22.06.2019 14:10
Precision can be defined as the o exact center of a data set. o reproducibility of a measured value. o correlation between two variables that are measured in a data set agreement between a measured value and an accepted value.
Answers: 2

Chemistry, 22.06.2019 19:20
The equation picture below shows which type of nuclear reaction u 235 + n x e 134 + sr 100 + 2n
Answers: 1
You know the right answer?
1) the equilibrium constant (keq) for the following reaction is 6.2x10-6. for the following assume a...
Questions

Mathematics, 15.10.2019 15:00

Mathematics, 15.10.2019 15:00




Biology, 15.10.2019 15:00





Mathematics, 15.10.2019 15:00

History, 15.10.2019 15:00

Mathematics, 15.10.2019 15:00

Biology, 15.10.2019 15:00

Computers and Technology, 15.10.2019 15:00

Mathematics, 15.10.2019 15:00


Social Studies, 15.10.2019 15:00

Chemistry, 15.10.2019 15:00

Mathematics, 15.10.2019 15:00

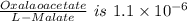
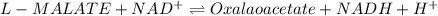
![\bigtriangleup G^{o}\,=\,-RTlnK\\=-8314\times 298ln\times 6.2\times 10^{-6}\\\=-2477.572[ln6.2+ln10^{-6}]\\=-5705.84[log6.2+log10^{-6}]\\=29729.77](/tpl/images/0393/5995/934dc.png)
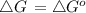
![-10=29.713+8.314\times298ln\frac{[Oxaloacetate][NADH]}{[L-Malate][NAD^{+}]}](/tpl/images/0393/5995/e7823.png)
![\frac{-39.713}{2.477}=2.303[log\frac{[oxaloacetate]}{[L-malate]}+log\frac{[NADH]}{NAD}]](/tpl/images/0393/5995/df966.png)
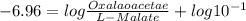
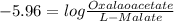
![\frac{[Oxalaoacetate]}{[L-Malate]}=1.1\times10^{-6}](/tpl/images/0393/5995/ff293.png)


