
Chemistry, 27.11.2019 20:31 hhvgbv7147
When 0.485 g of compound x is burned completely in a bomb calorimeter containing 3000 g of water, a temperature rise of 0.285°c is observed. what is δu of the reaction for the combustion of compound x? the hardware component of the calorimeter has a heat capacity of 3.81 kj/°c. the specific heat of water is 4.184 j/g·°c, and the mw of x is 56.0 g/mol.
a. -538 kj/mol
b. 4660 kj/mol
c. -4660 kj/mol
d. 538 kj/mol

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 04:20
Which formula can be used to calculate the molar mass of ammonia (nh3)? molar mass of n + molar mass of h 3 × molar mass of n + molar mass of h molar mass of n + 3 × molar mass of h 3 × molar mass of n + 3 × molar mass of h
Answers: 1

Chemistry, 22.06.2019 10:00
A50.0g sample of liquid water at 0.0 c ends up as ice at -20.0 c. how much energy is involved in this change?
Answers: 1

Chemistry, 23.06.2019 02:00
Anitrogen atom and an oxygen atom combine chemically to form nitric oxide. what is nitric oxide?
Answers: 1

You know the right answer?
When 0.485 g of compound x is burned completely in a bomb calorimeter containing 3000 g of water, a...
Questions

Mathematics, 26.03.2021 17:30


Mathematics, 26.03.2021 17:30


Mathematics, 26.03.2021 17:30



Computers and Technology, 26.03.2021 17:30

Physics, 26.03.2021 17:30


English, 26.03.2021 17:30


Spanish, 26.03.2021 17:30

Spanish, 26.03.2021 17:30





Mathematics, 26.03.2021 17:30

Mathematics, 26.03.2021 17:30

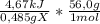 = -539kJ/mol ≡ a. -538 kJ/mol
= -539kJ/mol ≡ a. -538 kJ/mol

