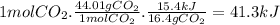Chemistry, 27.11.2019 19:31 eheheh80ii
The reaction of carbon dioxide(g) with hydrogen(g) to form carbon monoxide(g) and water(g) proceeds as follows: co2(g) + h2(g) > co(g) + h2o(g)when 16.4 grams of co2(g) react with sufficient h2(g) , 15.4 kj of energy areabsorbed .what is the value of > h for the chemical equation given? δhrxn = kj

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 03:40
In an effort to address concerns about global warming, a power plant in portland,oregon is designed to take all of its exhaust gases from its boilers and recycle the co2 using the solvay process to make sodium hydrogen carbonate. the reaction is shown below. nh3(g) + h2o(l) + co2(g) + nacl(aq) → nahco3(aq) + nh4cl(aq) how many liters each of nh3 and co2 (both at stp) would be consumed to produce 3.00 kg of sodium bicarbonate? the volume of both nh3 and co2 would be
Answers: 1

Chemistry, 22.06.2019 06:00
There are 6.022, 104 atoms of hg in 1 mole of hg the number of atoms in 45 moles of hg can be found by multiplying 4.5 by 6.022, 102 which is the number of atoms in 4.5 moles of hg, correctly written in scientific notation with the correct number of significant figures? 0 21,109 0 21,100 271, 1024 27.099, 100 mark this and retum save and exit submit
Answers: 1

Chemistry, 22.06.2019 10:00
Nonpoint source pollution is difficult to control because it
Answers: 2

Chemistry, 22.06.2019 15:30
Which suspect most likely committed the robbery and how do you know
Answers: 2
You know the right answer?
The reaction of carbon dioxide(g) with hydrogen(g) to form carbon monoxide(g) and water(g) proceeds...
Questions




Chemistry, 09.07.2021 20:50

Computers and Technology, 09.07.2021 20:50





Mathematics, 09.07.2021 20:50





Mathematics, 09.07.2021 20:50





Mathematics, 09.07.2021 20:50