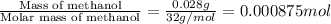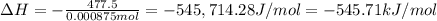Abomb calorimetric experiment was run to determine the enthalpy of combustion of methanol. the reaction is ch3oh(l)+3/2o2(g)→co2(g)+2h2o(l) the bomb calorimeter has a heat capacity of 250.0 j/k. burning 0.028 g of methanol resulted in a rise in temperature from 21.50 ∘c to 23.41 ∘c. calculate the change in internal energy for the combustion of methanol in kj/mol.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 06:00
The tilt of the earth's axis of rotation is responsible for the a) ocean's tides. b) size of the moon. c) brightness of stars. d) earth’s seasons.
Answers: 1


Chemistry, 22.06.2019 07:50
In which situation can a mixture always be called a solution
Answers: 3

Chemistry, 22.06.2019 09:30
Which element is the least metallic between cadmium, silver, zinc, or iron?
Answers: 1
You know the right answer?
Abomb calorimetric experiment was run to determine the enthalpy of combustion of methanol. the react...
Questions

Biology, 10.02.2021 18:00



Spanish, 10.02.2021 18:00


Mathematics, 10.02.2021 18:00





Advanced Placement (AP), 10.02.2021 18:00



Mathematics, 10.02.2021 18:00

English, 10.02.2021 18:00

Mathematics, 10.02.2021 18:00

Biology, 10.02.2021 18:00

Mathematics, 10.02.2021 18:00


Mathematics, 10.02.2021 18:00

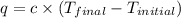
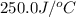
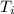 = Initial temperature =
= Initial temperature = 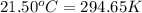
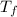 = Final temperature =
= Final temperature = 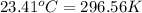
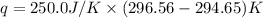
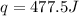
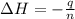
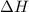 = enthalpy change = ?
= enthalpy change = ?