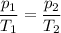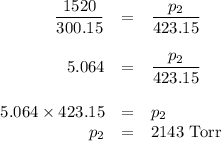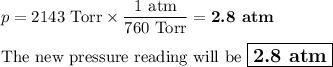Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 18:00
Alidded glass container is filled with a colored gas. after a period of time, it is observed that the gas is uniformly spread throughout the box and that the movement has slowed considerably. next, a warm iron plate is carefully placed under the box. why is there resumed movement of the gas in the container?
Answers: 2


Chemistry, 23.06.2019 15:30
How many grams of c3h8 is needed in the reactants to produce 10.5 mil of h2o
Answers: 2

Chemistry, 23.06.2019 15:30
Light travels through space at 186,282 miles per second and it takes about 1.3 seconds for light to travel from the moon to earth. which of the following is the correct method of finding the distance, in miles, between the moon and earth? add 186,282 and 1.3 divide 186,282 by 1.3 multiply 186,282 by 1.3 subtract 1.3 from 186,282
Answers: 1
You know the right answer?
Asample of n2 gas in a flask is heated from 27 celcius to 150 celcius. if the original gas is @ pres...
Questions


Arts, 02.11.2020 17:10

Mathematics, 02.11.2020 17:10








Spanish, 02.11.2020 17:10








Mathematics, 02.11.2020 17:10

Advanced Placement (AP), 02.11.2020 17:10