
Chemistry, 07.10.2019 01:00 tiffanybrown703
You dissolve 0.74 g of potassium chloride (kcl) in 500 ml of water. what is the molarity of the solution?
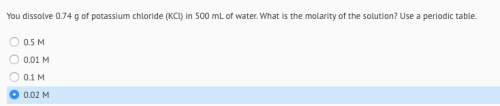

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 03:00
Schrodinger and heisenberg developed an alternate theory about atomic nature that contradicted some of bohr's model of the atom. how do changes resulting from new technology and evidence affect the reputation of the atomic theory?
Answers: 1

Chemistry, 22.06.2019 03:30
Explain why pure hydrogen cyanide does not conduct electricity, but become a conductor when it is dissolved in water? (at room temp, pure hcn exists as a volatile liquid)
Answers: 1

Chemistry, 22.06.2019 05:30
Which of the following signs of a chemical reaction are observed in the reaction of potassium with water? precipitate formed temperature change smell produced gas produced color change
Answers: 2

Chemistry, 22.06.2019 09:20
What happened to the amount of carbon dioxide in the atmosphere from 2010–2017?
Answers: 1
You know the right answer?
You dissolve 0.74 g of potassium chloride (kcl) in 500 ml of water. what is the molarity of the solu...
Questions

Advanced Placement (AP), 29.03.2021 17:20


French, 29.03.2021 17:20

Engineering, 29.03.2021 17:20


Mathematics, 29.03.2021 17:30

Chemistry, 29.03.2021 17:30

Mathematics, 29.03.2021 17:30




Mathematics, 29.03.2021 17:30



Mathematics, 29.03.2021 17:30



Mathematics, 29.03.2021 17:30

English, 29.03.2021 17:30

Mathematics, 29.03.2021 17:30



