
Chemistry, 27.11.2019 04:31 johnny2585
What is the theoretical yield of vanadium, in moles, that can be produced by the reaction of 1.0 mole of v2o5 with 4.0 moles of calcium based on the following chemical equation? v2o5(s) + 5ca(l) → 2v(l) + 5cao(s)

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 17:40
If 10.0 ml of the solution on the right are withdrawn from the 100 ml beaker and diluted again in a similar manner, what is the new concentration? m nacl
Answers: 2

Chemistry, 21.06.2019 22:20
Asolution is made by dissolving 25.5 grams of glucose (c6h12o6) in 398 grams of water. what is the freezing point depression of the solvent if the freezing point constant is -1.86 °c/m? show all of the work needed to solve this problem.
Answers: 1

Chemistry, 22.06.2019 04:00
Tin has ten stable isotopes. the heaviest, 124sn, makes up 5.80% of naturally occuring tin atoms. how many atoms of 124sn are present in 82.0 g of naturally occurring tin? what is the total mass of the 124sn atoms in this sample?
Answers: 3

Chemistry, 22.06.2019 14:00
What mass of natural gas (ch4) must you burn to emit 276 kj of heat?
Answers: 1
You know the right answer?
What is the theoretical yield of vanadium, in moles, that can be produced by the reaction of 1.0 mol...
Questions

English, 21.09.2020 09:01

History, 21.09.2020 09:01


Chemistry, 21.09.2020 09:01

Business, 21.09.2020 09:01

Chemistry, 21.09.2020 09:01

History, 21.09.2020 09:01


Mathematics, 21.09.2020 09:01


Advanced Placement (AP), 21.09.2020 09:01

Mathematics, 21.09.2020 09:01

Mathematics, 21.09.2020 09:01


Mathematics, 21.09.2020 09:01



Mathematics, 21.09.2020 09:01


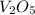 = 1.0 moles
= 1.0 moles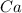 = 4.0 moles
= 4.0 moles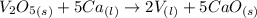
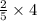 mole of V
mole of V

