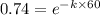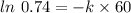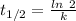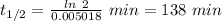Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 10:00
Water's surface tension and heat storage capacity are accounted for by its a) orbitals b) weight c) hydrogen bonds d) mass e) size
Answers: 2

Chemistry, 22.06.2019 11:00
What is the temperature of 0.750 mol of a gas stored in a 6,850 ml cylinder at 2.21 atm? . 2.95 k 5.24 k 138 k 246 k
Answers: 3

Chemistry, 22.06.2019 16:00
How will the volume of a gas be affected if the pressure is tripled, but the temperature remains the same?
Answers: 3

Chemistry, 22.06.2019 18:10
Given the following at 25c calculate delta hf for hcn (g) at 25c. 2nh3 (g) +3o2 (g) + 2ch4 (g) > 2hcn (g) + 6h2o (g) delta h rxn= -870.8 kj. delta hf=-80.3 kj/mol for nh3 (g), -74.6 kj/mol for ch4, and -241.8 kj/mol for h2o (g)
Answers: 1
You know the right answer?
Acompound decomposes by a first-order process. if 26 of the compound decomposes in 60 minutes, the h...
Questions

Mathematics, 16.10.2020 16:01

History, 16.10.2020 16:01





History, 16.10.2020 16:01


Mathematics, 16.10.2020 16:01

Mathematics, 16.10.2020 16:01


Mathematics, 16.10.2020 16:01

History, 16.10.2020 16:01

English, 16.10.2020 16:01

History, 16.10.2020 16:01

English, 16.10.2020 16:01


History, 16.10.2020 16:01

History, 16.10.2020 16:01

![[A_t]=[A_0]e^{-kt}](/tpl/images/0392/7747/1ef89.png)
![[A_t]](/tpl/images/0392/7747/5262c.png) is the concentration at time t
is the concentration at time t
![[A_0]](/tpl/images/0392/7747/9a686.png) is the initial concentration
is the initial concentration
![\frac {[A_t]}{[A_0]}](/tpl/images/0392/7747/0d33c.png) = 1 - 0.26 = 0.74
= 1 - 0.26 = 0.74
![\frac {[A_t]}{[A_0]}=e^{-k\times t}](/tpl/images/0392/7747/16cf4.png)