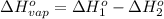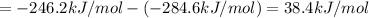Chemistry, 27.11.2019 03:31 KariSupreme
At a given set of conditions, 246.2 kj is given off when 1 mol of h2o(g) forms from its elements. under the same conditions, 284.6 kj is given off when 1 mol of h2o(l) forms from its elements. find δh for the vaporization of water at these conditions.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Asample of neon occupies a volume of 375 ml at stp. what will be the volume of neon if the pressure is reduced to 90.0 kpa? a. 422 ml b. 422 l c. 333 ml d. 333 l
Answers: 2

Chemistry, 22.06.2019 00:30
This active feature of earth's crust in building mountain ranges as well as islands. this feature is a a) cavern. b) earthquake. c) mountain. d) volcano.
Answers: 2

Chemistry, 22.06.2019 11:30
Voltaic cells produce a positive overall charge. what does this indicate? a. the reaction is likely to be endothermic. b. the reaction is spontaneous. c. the reaction is not likely to occur. d. the reaction is not spontaneous.
Answers: 3

Chemistry, 22.06.2019 15:30
Each of the following reactions is allowed to come to equilibrium and then the volume is changed as indicated. predict the effect (shift right, shift left, or no effect) of the indicated volume change. drag the appropriate items to their respective bins.co(g) + h2o(g) < => co2(g) + h2(g) (volume is decreased) pcl3(g) + cl2(g) < => pcl5(g) (volume is increased) caco3(s)< => cao(s) + co2(g) (volume is increased)
Answers: 1
You know the right answer?
At a given set of conditions, 246.2 kj is given off when 1 mol of h2o(g) forms from its elements. un...
Questions

Health, 10.07.2021 20:10

Mathematics, 10.07.2021 20:10

Mathematics, 10.07.2021 20:10


Mathematics, 10.07.2021 20:10

Mathematics, 10.07.2021 20:10



Mathematics, 10.07.2021 20:10

Mathematics, 10.07.2021 20:10



Chemistry, 10.07.2021 20:20

Mathematics, 10.07.2021 20:20






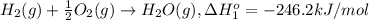 ..[1]
..[1]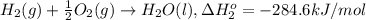 ..[2]
..[2]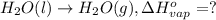 ...[3]
...[3]