Answers: 1


Another question on Chemistry


Chemistry, 21.06.2019 20:30
Which of the following true? a_volcanoes and earthquakes often near the plate boundaries. b_volcanoes occur whereve there are tall mountains. c_earthquakes cause volcanoes in the same location to erupt violently d_volcanoes and earthquakes occur only where plates are colliding with each other
Answers: 2

Chemistry, 22.06.2019 05:30
Compare and contrast physical changes with chemical changes.
Answers: 1

Chemistry, 22.06.2019 14:10
13. a covalent bond between two atoms is likely to be polar if: a. one of the atoms is much more electronegative than the other. b. the two atoms are equally electronegative. c. the two atoms are of the same element. d. the bond is part of a tetrahedrally shaped molecule. e. one atom is an anion.
Answers: 1
You know the right answer?
Determine the energy of 2.00 mol of photons for each of the following kinds of light. (assume three...
Questions








Mathematics, 23.10.2019 00:30


Computers and Technology, 23.10.2019 00:30


History, 23.10.2019 00:30


Computers and Technology, 23.10.2019 00:30



Mathematics, 23.10.2019 00:30



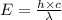
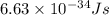
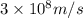
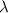 = wavelength =
= wavelength = 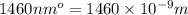
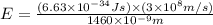
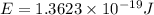
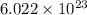 particles
particles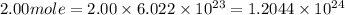 photons
photons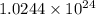 photons = E'
photons = E'