
Chemistry, 27.11.2019 02:31 merrymary3000
The balanced equation for the reaction of bromate ion with bromide in acidic solution is  at a particular instant in time, the rate of disappearance of br– is 2.0 x 10⁻³ mol/l • s. what is the rate of appearance of br₂ at the same instant?
at a particular instant in time, the rate of disappearance of br– is 2.0 x 10⁻³ mol/l • s. what is the rate of appearance of br₂ at the same instant?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 18:30
The number of moles of a given mass of a substance can be found without knowing its molecular formula or molar mass. true false
Answers: 1

Chemistry, 22.06.2019 19:30
Anurse used a 0.02-mg/l solution of disinfection to clean a patients wound. what is the concentration of the solution expressed as a percentage?
Answers: 1

Chemistry, 23.06.2019 04:40
6) (a) calculate the absorbance of the solution if its concentration is 0.0278 m and its molar extinction coefficient is 35.9 l/(mol cm). the depth of the cell is 5 mm. (b) what is the %t? (7) calculate the absorbance of the solution if the transmitted light intensity is 70% of the initial light beam intensity
Answers: 1

Chemistry, 23.06.2019 11:30
How do you calculate the mass of a product when the amounts of more than one reactant are given?
Answers: 3
You know the right answer?
The balanced equation for the reaction of bromate ion with bromide in acidic solution is [tex]bro^-...
Questions

Mathematics, 26.08.2020 05:01

Mathematics, 26.08.2020 05:01

Mathematics, 26.08.2020 05:01











Advanced Placement (AP), 26.08.2020 05:01




Mathematics, 26.08.2020 05:01


Mathematics, 26.08.2020 05:01

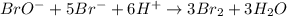
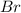 :
: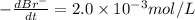
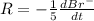
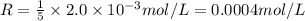
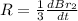
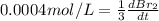
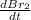 =3\times 0.0004 mol/L=0.0012 mol/L[/tex]
=3\times 0.0004 mol/L=0.0012 mol/L[/tex]

