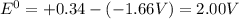Tarnish on copper is the compound cuo. a tarnished copper plate is placed in an aluminum pan of boiling water. when enough salt is added so that the solution conducts electricity, the tarnish disappears. imagine that the two halves of this redox reaction were separated and connected with a wire and a salt bridge.
calculate the standard cell potential given the following standard reduction potentials:
al3++3e-? al; e? =? 1.66 v
cu2++2e-? cu; e? =0.340 v

Answers: 2


Another question on Chemistry

Chemistry, 20.06.2019 18:04
Why is it illegal to manufacture fireworks without a license
Answers: 1

Chemistry, 22.06.2019 01:30
Agas is contained in a thick walled balloon when the pressure changes from 1.21 atm to 2.52 the volume changes from 3.75 l to 1.72 l and the temperature change from 293k to blank k
Answers: 3

Chemistry, 22.06.2019 08:40
Which statement can best be concluded from the ideal gas law?
Answers: 2

Chemistry, 22.06.2019 12:40
In the following table, all the columns for the element calcium are filled out correctly. element electron structure of atom electron structure of ion net ionic charge calcium 1s22s22p63s23p64s2 1s32s22p63s23p64s1 +1 true false
Answers: 2
You know the right answer?
Tarnish on copper is the compound cuo. a tarnished copper plate is placed in an aluminum pan of boil...
Questions



Mathematics, 27.08.2019 17:40


History, 27.08.2019 17:40


Biology, 27.08.2019 17:40




History, 27.08.2019 17:40



Mathematics, 27.08.2019 17:40

Mathematics, 27.08.2019 17:40


History, 27.08.2019 17:40



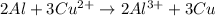

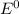 are standard reduction potentials.
are standard reduction potentials.
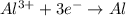
![E^0_{[Al^{3+}/Al]}=-1.66V](/tpl/images/0392/4616/0867c.png)
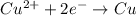
![E^0_{[Cu^{2+}/Cu]}=0.340V](/tpl/images/0392/4616/901e2.png)
![E^0=E^0_{[Cu^{2+}/Cu]}- E^0_{[Al^{3+}/Al]}](/tpl/images/0392/4616/78cb0.png)