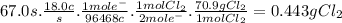Chemistry, 27.11.2019 01:31 ashhull2002
In the industrial "chlor-alkali" process, pure chlorine and sodium hydroxide are produced by electrolyzing brine, essentially an aqueous solution of sodium chloride.
suppose a current of 18.0 a is passed through an aqueous solution of nacl for 67.0 seconds.
calculate the mass of pure chlorine produced.
be sure your answer has a unit symbol and the correct number of significant digits.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 03:10
Describe the difference between a. a hypothesis and a theory and b. an observation and an experiment.
Answers: 1


Chemistry, 22.06.2019 08:00
Why is the bond angle in a water molecule less than the bond angle of methane? a. the central oxygen atom in water has two lone pairs of electrons, whereas the central carbon atom in methane has no lone pairs. b. the central hydrogen atom in water has one lone pair of electrons, whereas the central carbon atom in methane has two lone pairs. c. the central oxygen atom in water has four lone pairs of electrons, whereas the central carbon atom in methane has only one lone pair. d. the central oxygen atom exerts more repulsive force on surrounding atoms than the central carbon atom in methane does. reset next
Answers: 2

Chemistry, 23.06.2019 05:00
C=59(f−32)the equation above shows how temperature f, measured in degrees fahrenheit, relates to a temperature c, measured in degrees celsius. based on the equation, which of the following must be true? a temperature increase of 1 degree fahrenheit is equivalent to a temperature increase of 59 degree celsius.a temperature increase of 1 degree celsius is equivalent to a temperature increase of 1.8 degrees fahrenheit.a temperature increase of 59 degree fahrenheit is equivalent to a temperature increase of 1 degree celsius.a) i onlyb) ii onlyc) iii onlyd) i and ii only
Answers: 1
You know the right answer?
In the industrial "chlor-alkali" process, pure chlorine and sodium hydroxide are produced by electro...
Questions


Physics, 26.07.2019 23:20

Law, 26.07.2019 23:20

Mathematics, 26.07.2019 23:20



Mathematics, 26.07.2019 23:20

Mathematics, 26.07.2019 23:20

History, 26.07.2019 23:20

Mathematics, 26.07.2019 23:20

History, 26.07.2019 23:20

Mathematics, 26.07.2019 23:20


Mathematics, 26.07.2019 23:20

Mathematics, 26.07.2019 23:20


Computers and Technology, 26.07.2019 23:20

Mathematics, 26.07.2019 23:20

English, 26.07.2019 23:20