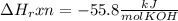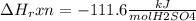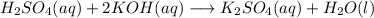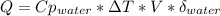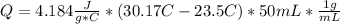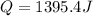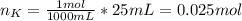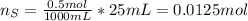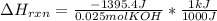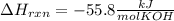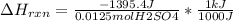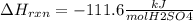When 25.0 ml of 0.500 m h2so4 is added to 25.00 mk of 1.00 m koh in a coffee-cup calorimeter at 23.50◦ c, the temperature rises to 30.17◦ c. calculate dhrxn for this reaction. (assume that the density of water of and specific heat capacity of the solution are the same as for pure water). ( –55.8 kj/mol koh, –112 kj/mol h2so4)

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 07:30
Which of the following best supports the concept that genetic information is passed on to offspring from both of their parents, not just one?
Answers: 2

Chemistry, 22.06.2019 14:00
Will mark brainliest how many electrons can be held in the energy level n = 4?
Answers: 1

Chemistry, 22.06.2019 14:30
How can carbon move from "land" to bodies of water? describe the way human impact has lead to increased levels of co2 in the atmosphere.
Answers: 2

Chemistry, 22.06.2019 16:00
No copying 15 pts how does a free-body diagram tell you about the net force on an object?
Answers: 2
You know the right answer?
When 25.0 ml of 0.500 m h2so4 is added to 25.00 mk of 1.00 m koh in a coffee-cup calorimeter at 23.5...
Questions

Mathematics, 18.09.2021 23:40

Physics, 18.09.2021 23:40

World Languages, 18.09.2021 23:40






History, 18.09.2021 23:50

Mathematics, 18.09.2021 23:50



Mathematics, 18.09.2021 23:50

Mathematics, 18.09.2021 23:50

Mathematics, 18.09.2021 23:50


Chemistry, 18.09.2021 23:50

Geography, 18.09.2021 23:50


Social Studies, 18.09.2021 23:50