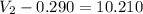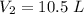Chemistry, 26.11.2019 21:31 athenajames1221
Afuel-air mixture is placed in a cylinder fitted with a piston. the original volume is 0.290-l. when the mixture is ignited, gases are produced and 865 j of energy is released. to what volume will the gases expand against a constant pressure of 635 mmhg, if all the energy released is converted to work to push the piston?
the answer is 10.5 l

Answers: 3


Another question on Chemistry


Chemistry, 21.06.2019 21:00
Harvey kept a balloon with a volume of 348 milliliters at 25.0˚c inside a freezer for a night. when he took it out, its new volume was 322 milliliters, but its pressure was the same. if the final temperature of the balloon is the same as the freezer’s, what is the temperature of the freezer? the temperature of the freezer is kelvins.
Answers: 2

Chemistry, 22.06.2019 00:30
What is the most stable monatomic ion formed from nitrogen
Answers: 2

Chemistry, 22.06.2019 05:00
Use the table to identify the phase and phase changes of the elements under the given conditions. write the name of the substance, phase, or phase change
Answers: 3
You know the right answer?
Afuel-air mixture is placed in a cylinder fitted with a piston. the original volume is 0.290-l. when...
Questions



History, 02.10.2019 19:30

Spanish, 02.10.2019 19:30


Spanish, 02.10.2019 19:30


Chemistry, 02.10.2019 19:30



Mathematics, 02.10.2019 19:30

Social Studies, 02.10.2019 19:30

Mathematics, 02.10.2019 19:30

Mathematics, 02.10.2019 19:30

Physics, 02.10.2019 19:30


Mathematics, 02.10.2019 19:30

Social Studies, 02.10.2019 19:30

Mathematics, 02.10.2019 19:30

Social Studies, 02.10.2019 19:30

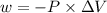
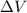 is the change in volume
is the change in volume
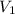 = 0.290 L
= 0.290 L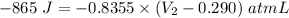
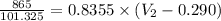
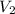 , we get that:-
, we get that:-