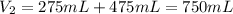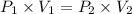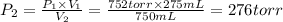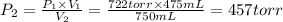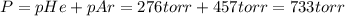A275-ml flask contains pure helium at a pressure of 752 torr. a second flask with a volume of 475 ml contains pure argon at a pressure of 722 torr. if the two flasks are connected through a stopcock and the stopcock is opened, what is the partial pressure of each gas and the total pressure.?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 02:50
Using a value of ksp = 1.8 x 10-2 for the reaction pbcl2 pb+2(aq) + 2cl -(aq). if the value of ksp was determined to be only 1.2 x 10-2: too much solid has dissolved. additional precipitate is forming. the solution is unsaturated. the ions are now combining to reduce their concentrations.
Answers: 3

Chemistry, 22.06.2019 12:00
Which of the following units is not an official si unit? mole liter kilogram ampere
Answers: 1

Chemistry, 22.06.2019 15:30
Count the number of each type of atom in the equation below, and then balance the equation. write in the numbers of atoms and coefficients. add a 1 if there should be no coefficient. cs2(l) + o2(g) → co2(g) + so2(g) c [ ] s [ ] o > c [ ] s [ ] o [ ] cs2(l) + [ ] o2(g) > [ ] co2(g) + [ ] so2(g)
Answers: 3

You know the right answer?
A275-ml flask contains pure helium at a pressure of 752 torr. a second flask with a volume of 475 ml...
Questions


Biology, 19.07.2019 16:00

Biology, 19.07.2019 16:00

Chemistry, 19.07.2019 16:00


Biology, 19.07.2019 16:00

Mathematics, 19.07.2019 16:00


Biology, 19.07.2019 16:00

Mathematics, 19.07.2019 16:00



Health, 19.07.2019 16:00




History, 19.07.2019 16:00


Geography, 19.07.2019 16:00

Geography, 19.07.2019 16:00