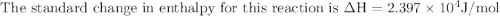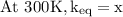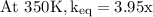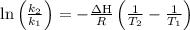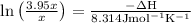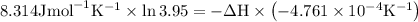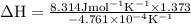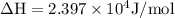Answers: 1


Another question on Chemistry


Chemistry, 23.06.2019 07:30
Which of the following statements best explains why chemistry is testable a) it can measure data by experiments b) it cannot add new evidence c) it cannot be verified d) it is biased
Answers: 1

Chemistry, 23.06.2019 10:30
Amethod of separation that employs a system with two phases of matter, a mobile phase and a stationary phase, is called
Answers: 2

You know the right answer?
The equilibrium constant for a certain reaction increases by a factor of 3.95 when the temperature i...
Questions



Physics, 22.06.2019 07:00


Mathematics, 22.06.2019 07:00