
Chemistry, 26.11.2019 07:31 emanuelmorales1515
The ksp for zn(oh)2 is 5.0 x 10-17. determine the molar solubility of zn(oh)2 in a buffer solution with a ph of 11.5.
a) 5.0 x 106
b) 1.2 x 10-12
c) 1.6 x 10-14
d) 5.0 x 10-12
e) 5.0 x 10-17

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 17:00
What were the success and failures that came to boyle’s excitements?
Answers: 1

Chemistry, 22.06.2019 03:20
What is the ima of the 1 st class lever in the graphic given? 2 3 0.5
Answers: 1

Chemistry, 22.06.2019 03:30
If a solution is considered basic, then a) the hydroxide ion and hydronium ion concentrations are equal. b) the hydroxide ion concentration is less than the hydronium ion concentration. c) the hydronium ion concentration is greater than the hydroxide ion concentration. d) the hydroxide ion concentration is greater than the hydronium ion concentration.
Answers: 1

Chemistry, 22.06.2019 22:30
What is the value of the standard enthalpy of formation of an element in its most stable form?
Answers: 3
You know the right answer?
The ksp for zn(oh)2 is 5.0 x 10-17. determine the molar solubility of zn(oh)2 in a buffer solution w...
Questions

Mathematics, 29.01.2021 01:00

Mathematics, 29.01.2021 01:00


English, 29.01.2021 01:00

History, 29.01.2021 01:00

Mathematics, 29.01.2021 01:00


Chemistry, 29.01.2021 01:00


Biology, 29.01.2021 01:00

Mathematics, 29.01.2021 01:00

History, 29.01.2021 01:00


Chemistry, 29.01.2021 01:00

Chemistry, 29.01.2021 01:00


English, 29.01.2021 01:00

Chemistry, 29.01.2021 01:00

Arts, 29.01.2021 01:00

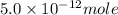
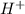 concentration.
concentration.![pH=-\log [H^+]](/tpl/images/0391/2894/37e81.png)
![11.5=-\log [H^+]](/tpl/images/0391/2894/f3755.png)
![[H^+]=3.16\times 10^{-12}M](/tpl/images/0391/2894/3012d.png)
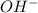 concentration.
concentration.![[H^+][OH^-]=K_w](/tpl/images/0391/2894/55f9c.png)
![3.16\times 10^{-12}\times [OH^-]=1.0\times 10^{-14}](/tpl/images/0391/2894/64b28.png)
![[OH^-]=3.16\times 10^{-3}M](/tpl/images/0391/2894/38f53.png)
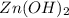 .
.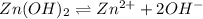
![K_{sp}=[Zn^{2+}][OH^-]^2](/tpl/images/0391/2894/b302a.png)
![5.0\times 10^{-17}=[Zn^{2+}]\times (3.16\times 10^{-3})^2](/tpl/images/0391/2894/0b771.png)
![[Zn^{2+}]=5.0\times 10^{-12}M](/tpl/images/0391/2894/e914d.png)


