
Chemistry, 26.11.2019 06:31 jdkrisdaimcc11
The thiocyanate polyatomic ion, scn-, is commanly called a pseudohalogen because it acts very much like halide ions. for example, we know that pure halogens consists of diatiomic molecules, such as c12. thiocanate ions form similar molecules in the following reaction2nascn +2h2so4 + mno2 -> (scn)2 + 2h2o + mnso4 + na2so4a) write a conversion factor that could be used to convert between moles of nascn and moles of (scn)2b) how many moles of (scn)2 form when 0.05 moles of nascn react completely? c) what is the maximum number of moles of (scn)2 that could form in the combination of 4 moles of nascn and 3 moles of mnso4? d) write a convertion factor that could be used to convert between moles of sulfuric acid, h2so4 and moles manganese (ii) sulfate, mnso4.e) what is the minimum number of moles of h2so4 that must react to form 1.7752 moles of manganese (ii) sulfate?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 18:40
What is the binding energy of a nucleus that has a mass defect of 5.81*10-^29 kg a 5.23*10-^12 j b 3.15* 10^12 j c 1.57*10-3 j d 9.44*10^20 j
Answers: 1


Chemistry, 23.06.2019 10:50
Achemist reacted 57.50 grams of sodium metal with an excess amount of chlorine gas. the chemical reaction that occurred is shown. na + cl2 → nacl if the percentage yield of the reaction is 86%, what is the actual yield? show your work, including the use of stoichiometric calculations and conversion factors.
Answers: 1

You know the right answer?
The thiocyanate polyatomic ion, scn-, is commanly called a pseudohalogen because it acts very much l...
Questions


Physics, 25.08.2019 23:00


Mathematics, 25.08.2019 23:00

Health, 25.08.2019 23:00

Mathematics, 25.08.2019 23:00



History, 25.08.2019 23:00

Mathematics, 25.08.2019 23:00

Mathematics, 25.08.2019 23:00



Business, 25.08.2019 23:00

History, 25.08.2019 23:00

Mathematics, 25.08.2019 23:00

Biology, 25.08.2019 23:00

Biology, 25.08.2019 23:00

Mathematics, 25.08.2019 23:00

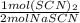
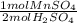
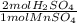 = 3.5504 mol H₂SO₄
= 3.5504 mol H₂SO₄

