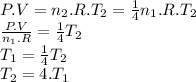Chemistry, 26.11.2019 06:31 kinziemadison12
Achemist prepares a sample of helium gas at a certain pressure, temperature and volume and then removes all but a fourth of the gas molecules (only a fourth remain). how must the temperature be changed (as a multiple of t1) to keep the pressure and the volume the same? a. t2=1/16t1b. t2=2t1c. t2=16t1d. t2= 1/2t1e. t2=4t1f. none of theseg. t2=1/4t1

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 09:00
Scientific evidence tells us that the cause of earths four season is the tilt of earth as it revolves around the sun. the student is instructed to illustrate this information in a science notebook. how will the student illiterate winter in the northern hemisphere?
Answers: 3

Chemistry, 22.06.2019 16:30
For the reaction shown, calculate how many moles of no2 form when each of the following completely reacts. 2n2o5(g)→4no2(g)+o2(g) part a 1.0 mol n2o5 express your answer using two significant figures. nothing mol m o l request answer part b 5.4 mol n2o5 express your answer using two significant figures.
Answers: 2

Chemistry, 22.06.2019 19:40
What is the wavelength of a 3*10^12 hz infrared wave a 3*10^20m b 1* 10^4m c 3*10^-3m d 1*10^-4 m
Answers: 1

Chemistry, 23.06.2019 06:00
In an exothermic reaction at equilibrium, what is the effect of lowering the temperature? a. the reaction makes more products. b. the reaction makes more reactants. c. the reaction is unchanged.
Answers: 1
You know the right answer?
Achemist prepares a sample of helium gas at a certain pressure, temperature and volume and then remo...
Questions


Mathematics, 25.07.2019 23:30









Mathematics, 25.07.2019 23:30


Mathematics, 25.07.2019 23:30


Physics, 25.07.2019 23:30