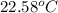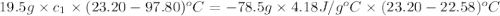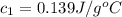Chemistry, 26.11.2019 06:31 JaleahOwens13
In the laboratory a student uses a "coffee cup" calorimeter to determine the specific heat of a metal.
she heats 19.5 grams of tungsten to 97.80°c and then drops it into a cup containing 78.3 grams of water at 22.58°c. she measures the final temperature to be 23.20°c.
assuming that all of the heat is transferred to the water, she calculates the specific heat of tungsten to be j/g°c.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 00:00
Which of the following methods uses the decay of atomic particles in an object to find its exact age? a. fossil dating b. geologic dating c. radioactive dating d. relative dating
Answers: 1

Chemistry, 22.06.2019 05:00
If the density of water is 1.0 g/cm3, which of these materials would float in water, based on their densities? check all that apply. aluminum cork iron lead wax
Answers: 1

Chemistry, 22.06.2019 05:00
What forms when chemical reactions combine pollution with sunlight?
Answers: 1

Chemistry, 22.06.2019 07:30
11. phosphorus-32 is radioactive and has a half life of 14 days. how much of a 124 mg sample of phosphorus-32 is present after 56 days? a) 7.75 mg b) 15.5 mg c) 31.0 mg d) 62.0 mg
Answers: 3
You know the right answer?
In the laboratory a student uses a "coffee cup" calorimeter to determine the specific heat of a meta...
Questions


Chemistry, 19.09.2021 14:00


Geography, 19.09.2021 14:00

Mathematics, 19.09.2021 14:00

Mathematics, 19.09.2021 14:00

Advanced Placement (AP), 19.09.2021 14:00

History, 19.09.2021 14:00

Mathematics, 19.09.2021 14:00

Biology, 19.09.2021 14:00






Social Studies, 19.09.2021 14:00


Mathematics, 19.09.2021 14:00


Social Studies, 19.09.2021 14:00

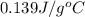
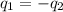
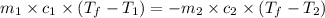
 = specific heat of tungsten = ?
= specific heat of tungsten = ? = specific heat of water =
= specific heat of water = 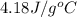
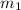 = mass of tungsten = 19.5 g
= mass of tungsten = 19.5 g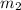 = mass of water = 78.5 g
= mass of water = 78.5 g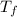 = final temperature =
= final temperature = 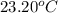
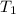 = initial temperature of tungsten =
= initial temperature of tungsten = 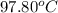
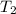 = initial temperature of water =
= initial temperature of water =