

Answers: 3


Another question on Chemistry

Chemistry, 23.06.2019 00:00
The empirical formula of a compound is ch2o and its mass is 120 amu/molecule, what is its formula?
Answers: 1

Chemistry, 23.06.2019 07:00
0.88 moles of n2o5 (g) was placed in a sealed 1.00 l vessel. calculate the equilibrium concentration of n2o5. no2, and o2 and the equilibrium constant after equilibrium has been reached by 65.0% of the n2o5 decomposing.
Answers: 1

Chemistry, 23.06.2019 13:30
1. what is boyle’s law? • state the definition of the law in words. • what are the assumptions of boyle’s law? • write at least one mathematical equation that represents the law. • what can be calculated with boyle’s law? • using a gas-filled balloon as an example, describe what is happening to the gas molecules inside the balloon before and after you squeeze it.
Answers: 2

Chemistry, 23.06.2019 14:00
How does electronegativity changes as we move from left to right across a period
Answers: 2
You know the right answer?
Given the standard enthalpy changes for the following two reactions:
(1) n2(g) + 2o2(g)2no2(...
(1) n2(g) + 2o2(g)2no2(...
Questions







History, 27.02.2020 05:44






Mathematics, 27.02.2020 05:45


Mathematics, 27.02.2020 05:45





English, 27.02.2020 05:45

 ...... ΔH° = 66.4 kJ
...... ΔH° = 66.4 kJ
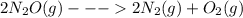 ......ΔH° = -164.2 kJ
......ΔH° = -164.2 kJ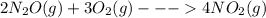 ......ΔH° = _________?
......ΔH° = _________?

