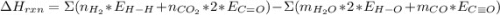Chemistry, 26.11.2019 05:31 marissalwilliams3
Use bond energies to calculate the enthalpy change for the following reaction. h2(g) + co2(g) ⟶ h2o(g) + co(g)

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 09:40
Which diagram shows the correct way to represent an ionic compound of magnesium oxide?
Answers: 3

Chemistry, 22.06.2019 13:00
In what environment would mineral formation caused by high pressures and high temperatures most likely occur?
Answers: 3

Chemistry, 22.06.2019 17:10
Some liquids can be distilled, but only at temperatures that are so high that it is impractical, or so high the compound decomposes. explain why distillation such compounds at significantly less than atmospheric pressure (some degree of vacuum) would solve this problem.
Answers: 2

Chemistry, 22.06.2019 22:00
The volume of an unknown substance in a sealed glass jar is 50 milliliters. the volume of the jar is 200 milliliters. which state of matter could the substance be?
Answers: 2
You know the right answer?
Use bond energies to calculate the enthalpy change for the following reaction. h2(g) + co2(g) ⟶ h2o(...
Questions










Computers and Technology, 12.08.2020 05:01










English, 12.08.2020 05:01

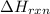 ) can be calculated with the next equation:
) can be calculated with the next equation: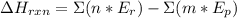
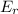 and
and 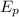 are the bond energies of the reactants and the products, respectively
are the bond energies of the reactants and the products, respectively