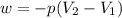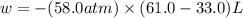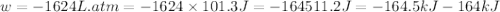Chemistry, 26.11.2019 04:31 tanakamkuzha
Question 22 a mixture of krypton and argon gas is expanded from a volume of 33.0l to a volume of 61.0l , while the pressure is held constant at 58.0atm . calculate the work done on the gas mixture. be sure your answer has the correct sign (positive or negative) and the correct number of significant digits.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 10:50
Determine the empirical formula for succinic acid that is composed of 40.60% carbon, 5.18% hydrogen, and 54.22% oxygen.
Answers: 1

Chemistry, 23.06.2019 11:20
Try to reduce the amount of leftover ingredients by changing the amount of one, two, or all three starting ingredients. show your stoichiometric calculations below. water 946.36 g sugar 196.86 g lemon juice193.37 g
Answers: 2

Chemistry, 23.06.2019 16:10
Which is the best term to use when describing the energy of position? chemical kinetic potential electromagnetic
Answers: 3

Chemistry, 23.06.2019 22:50
The sequence –accuuugaaacgac– provides the code for 18 amino acids 9 amino acids 6 amino acids 3 amino acids
Answers: 1
You know the right answer?
Question 22 a mixture of krypton and argon gas is expanded from a volume of 33.0l to a volume of 61....
Questions



Mathematics, 09.03.2021 02:40


Mathematics, 09.03.2021 02:40




Mathematics, 09.03.2021 02:40








Law, 09.03.2021 02:40


Mathematics, 09.03.2021 02:40

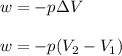
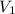 = initial volume = 33.0 L
= initial volume = 33.0 L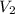 = final volume = 61.0 L
= final volume = 61.0 L