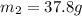Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 19:00
Asmall amount of a solid is added to water. the observation made after fifteen minutes is shown in the figure. which of these solids has been probably added to water? a) oil b) sand c) sugar d) wood chips
Answers: 1

Chemistry, 21.06.2019 20:40
You may expect bonds between two atoms which each have n covalent lonic metallic hydrogen
Answers: 2

Chemistry, 22.06.2019 04:00
What three natural resources are found in the great lakes region
Answers: 2

Chemistry, 22.06.2019 06:30
Three cards with holes are arranged in a straight line. a light is shined through the first card’s hole and travels through all three cards. what does this tell you about light rays? a) that light is reflected b) that light is refractive c) that light travels in a straight line d) that light does not travel in a straight line
Answers: 1
You know the right answer?
A32.4 g iron rod, initially at 21.6 ∘c, is submerged into an unknown mass of water at 63.1 ∘c, in an...
Questions





Mathematics, 05.03.2020 05:11

Health, 05.03.2020 05:11






Mathematics, 05.03.2020 05:12



Mathematics, 05.03.2020 05:12





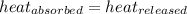
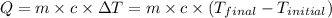
![m_1\times c_1\times (T_{final}-T_1)=-[m_2\times c_2\times (T_{final}-T_2)]](/tpl/images/0390/9808/09236.png) .................(1)
.................(1)
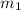 = mass of iron rod = 32.4 g
= mass of iron rod = 32.4 g
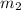 = mass of water = ?
= mass of water = ?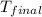 = final temperature =
= final temperature = 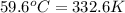
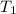 = temperature of iron rod =
= temperature of iron rod = 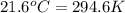
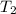 = temperature of water =
= temperature of water = 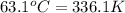
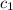 = specific heat of iron rod =
= specific heat of iron rod = 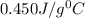
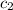 = specific heat of water=
= specific heat of water= 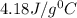
![32.4\times 0.450\times (332.6-294.6)=-[m_2\times 4.18\times (332.6-336.1)]](/tpl/images/0390/9808/cb847.png)