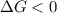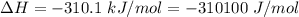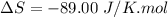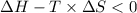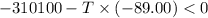Chemistry, 26.11.2019 03:31 Rileyb101207
Areaction will be spontaneous only at low temperatures if both δh and δs are negative. for a reaction in which δh = −310.1 kj/mol and δs = −89.00 j/k · mol, determine the temperature (in °c) below which the reaction is spontaneous.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 02:20
Brainliesttt me asap! the below reaction can be categorized as more than one type of reaction. which reactions are these, and what are the types of reactions?
Answers: 1

Chemistry, 22.06.2019 03:30
At a temperature of 393 k, the temperature of a sample of nitrogen is 1.07 atm what will the pressure be at a temperature of 478 k
Answers: 1

Chemistry, 22.06.2019 06:00
What type of electromagnetic radiation has a shorter wavelength than blue light
Answers: 2

Chemistry, 22.06.2019 06:00
How much would the freezing point of water decrease if 4 mol of sugar were added to 1 kg of water(k=1.86 c/mol/kg for water and i=1 for sugar
Answers: 1
You know the right answer?
Areaction will be spontaneous only at low temperatures if both δh and δs are negative. for a reactio...
Questions

Chemistry, 08.09.2019 23:10

Mathematics, 08.09.2019 23:10

Arts, 08.09.2019 23:10

Mathematics, 08.09.2019 23:10

Mathematics, 08.09.2019 23:10

Mathematics, 08.09.2019 23:10




Mathematics, 08.09.2019 23:10

Mathematics, 08.09.2019 23:10




Mathematics, 08.09.2019 23:10



History, 08.09.2019 23:10


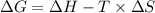
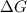 is the change in the Gibbs free energy.
is the change in the Gibbs free energy.
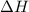 is the enthalpy change of the reaction.
is the enthalpy change of the reaction.
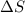 is the change in entropy.
is the change in entropy.