
Chemistry, 26.11.2019 03:31 aghamuzahirali5289
Aphosphate buffer solution (25.00 ml sample) used for a growth medium was titrated with 0.1000 m hydrochloric acid. the components of the buffer were sodium monohydrogenphosphate and sodium dihydrogenphosphate. the first endpoint occurred at a volume of 10.32 ml, and the second occurred after an additional 18.62 ml was added, for a total volume of 28.94 ml. what was the total concentration of phosphate (in any form) in the buffer?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 02:40
The difference between the atomic number of an element and the element’s atomic mass is the number of ions.
Answers: 3

Chemistry, 22.06.2019 12:30
Which of the following describes a compound? (hint: carbon and oxygen bo a. a piece of pure carbon, containing only carbon atoms b. oxygen gas surrounding a solid piece of carbon c. a substance made of two oxygen atoms for each carbon atom carbon and oxygen atoms mixed without being bonded together
Answers: 1


Chemistry, 23.06.2019 02:00
Pinene is an unsaturated hydrocarbon found in pine resin. if pinene has m+ = 136 and contains 1 double bond(s) and 2 ring(s); what is its molecular formula? enter the formula in the form ch first, then all other atoms in alphabetical order; do not use subscripts. the formula is case-sensitive
Answers: 3
You know the right answer?
Aphosphate buffer solution (25.00 ml sample) used for a growth medium was titrated with 0.1000 m hyd...
Questions


Mathematics, 31.05.2020 07:58


Mathematics, 31.05.2020 07:58


Mathematics, 31.05.2020 07:58



Mathematics, 31.05.2020 07:58

Mathematics, 31.05.2020 07:58



Mathematics, 31.05.2020 07:58

Mathematics, 31.05.2020 07:58




Mathematics, 31.05.2020 07:58

Mathematics, 31.05.2020 07:58

Business, 31.05.2020 07:58

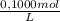 = 1,862x10⁻³moles of HCl ≡ moles of phosphate buffer.
= 1,862x10⁻³moles of HCl ≡ moles of phosphate buffer.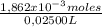 = 0,07448M of phosphate buffer
= 0,07448M of phosphate buffer

