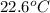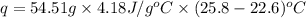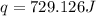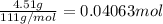Chemistry, 26.11.2019 03:31 juicecarton
When 4.51 g of cacl2 dissolved in 50.00 ml of water in a coffee cup calorimeter, the temperature of the solution rose from 22.6â°c to 25.8â°c.
specific heat of the solution is equal to the specific heat of water = 4.18 j/gâºc.
density of the solution is equal to the density of water = 1.00 g/ml.
what is qsolution?
what is qreaction ?
what is îhrxn in kj/mol of cacl2 ?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 17:30
I'm learning about the periodic tables and what each subject's configuration is. for example, hydrogen is 1s^1, but i don't understand how you get that. can someone me understand how to figure out how to figure this out? sorry if the question makes no sense, but it would really a lot if you could me understand! you so much if you can!
Answers: 1

Chemistry, 22.06.2019 20:00
I’m an electrically neutral atomic any element, there are equal numbers of
Answers: 2

Chemistry, 23.06.2019 08:30
Explain how to convert from one unit to another in the metric system.
Answers: 3

Chemistry, 23.06.2019 09:00
How many grams of ammonia are produced when 1.0 mole of nitrogen reacts
Answers: 2
You know the right answer?
When 4.51 g of cacl2 dissolved in 50.00 ml of water in a coffee cup calorimeter, the temperature of...
Questions


Biology, 16.10.2020 01:01


Mathematics, 16.10.2020 01:01

History, 16.10.2020 01:01







Mathematics, 16.10.2020 01:01





Mathematics, 16.10.2020 01:01

English, 16.10.2020 01:01

Chemistry, 16.10.2020 01:01

Mathematics, 16.10.2020 01:01

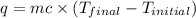
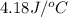
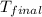 = final temperature =
= final temperature = 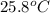
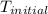 = initial temperature =
= initial temperature =