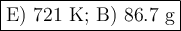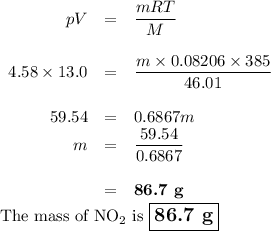So
25
7.) a syringe initially holds a sample of gas with a volume of 285 ml at 355 k and...

Chemistry, 26.11.2019 03:31 dpchill5232
So
25
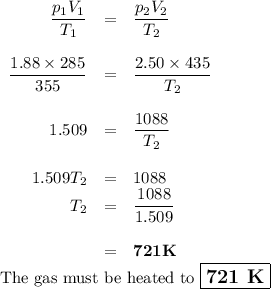
7.) a syringe initially holds a sample of gas with a volume of 285 ml at 355 k and 1.88 atm. to
what temperature must the gas in the syringe be heated/cooled in order to have a volume of 435
ml at 2.50 atm?
a) 139 k
b) 572 k
c) 175 k
d) 466 k
e) 721 k
8.) what mass of no2 is contained in a 13.0 l tank at 4.58 atm and 385 k?
a) 18.8 g
b) 86.7 g
c) 24.4 g
d) 53.1 g
e) 69.2 g

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 16:00
What is lincoln's purpose in writing this speech? question 1 options: to stress the difficulties of war to honor those who died in the war to call for an end to the war to call the country to join a new war
Answers: 1

Chemistry, 22.06.2019 06:00
Calculate the mass of silver needed to react with chlorine to produce 126g if silver chloride?
Answers: 3


Chemistry, 22.06.2019 23:10
Amines are good nucleophiles, even though they are neutral molecules. how would the rate of an sn2 reaction between an amine and an alkyl halide be affected if the polarity of the solvent is increased? amines are good nucleophiles, even though they are neutral molecules. how would the rate of an reaction between an amine and an alkyl halide be affected if the polarity of the solvent is increased? because both reactants in the rate-limiting step are neutral, the reaction will be faster if the polarity of the solvent is increased. because both reactants in the rate-limiting step are neutral, the reaction will be slower if the polarity of the solvent is increased. because both reactants in the rate-limiting step are neutral, the reaction will occur at the same rate if the polarity of the solvent is increased. request answer
Answers: 3
You know the right answer?
Questions


Computers and Technology, 04.03.2021 20:20

Computers and Technology, 04.03.2021 20:20

History, 04.03.2021 20:20



History, 04.03.2021 20:20



Chemistry, 04.03.2021 20:20


Mathematics, 04.03.2021 20:20

Mathematics, 04.03.2021 20:20

Spanish, 04.03.2021 20:20


Health, 04.03.2021 20:20



History, 04.03.2021 20:20