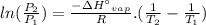Chemistry, 26.11.2019 02:31 zeesharpe05
Arsine, ash3, is a highly toxic compound used in the electronics industry for the production of semiconductors. its vapor pressure is 35 torr at 111.95 c and 253 torr at 83.6 c. using these data, calculate (a) the standard enthalpy of vaporization;

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 06:00
When a spring is compressed, the energy changes from kinetic to potential. which best describes what is causing this change?
Answers: 3



Chemistry, 22.06.2019 13:00
The molality of calcium chloride (cacl2) in an aqueous solution is 2.46 m. what is mole fraction of the solute?
Answers: 3
You know the right answer?
Arsine, ash3, is a highly toxic compound used in the electronics industry for the production of semi...
Questions




English, 28.07.2019 05:30


Physics, 28.07.2019 05:30


Mathematics, 28.07.2019 05:30






English, 28.07.2019 05:30

English, 28.07.2019 05:30



History, 28.07.2019 05:30


Mathematics, 28.07.2019 05:30