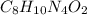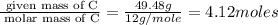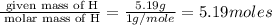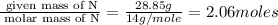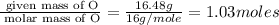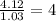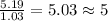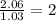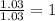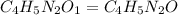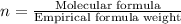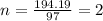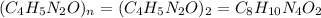Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 23:00
A100-watt light bulb radiates energy at a rate of 100 j/s. (the watt, a unit of power or energy over time, is defined as 1 j/s.) if all of the light emitted has a wavelength of 525 nm , how many photons are emitted per second?
Answers: 1

Chemistry, 22.06.2019 06:00
Calculate - analysis of compound composed of iron and oxygen yields 174.86 of fe and 75.14g of o. what is the empirical formula for this compound?
Answers: 3

Chemistry, 22.06.2019 08:30
Which part of earth’s surface receives the most direct rays from the sun? a) equator b) ocean c) poles d) mountains
Answers: 2

Chemistry, 22.06.2019 17:00
Astable electron arrangement for an atom is one that does not easily change. how is this arrangement arrived at? a. valence electrons are transferred or shared to create a full outer shell of electrons. b. valence electrons are discarded into space to create a full outer shell of electrons. c. protons (positive charge) pair with valence electrons (negative charge) to create a strong bond. d. outer shells with valence electrons are transferred or shared.
Answers: 2
You know the right answer?
Caffeine, a stimulant in coffee and tea, has a molar mass of 194.19 g/mol and a mass percentage comp...
Questions


Mathematics, 08.12.2020 20:30



Social Studies, 08.12.2020 20:30

Mathematics, 08.12.2020 20:30

Mathematics, 08.12.2020 20:30

Mathematics, 08.12.2020 20:30

Mathematics, 08.12.2020 20:30

Physics, 08.12.2020 20:30


Arts, 08.12.2020 20:30


History, 08.12.2020 20:30

Mathematics, 08.12.2020 20:30


History, 08.12.2020 20:30



Health, 08.12.2020 20:30