Chemistry, 26.11.2019 01:31 billlyyyyyyyyyy
Calculate the change internal energy (δe) for a system that is giving off 65.0 kj of heat and is performing 855 j of work on the surroundings. calculate the change internal energy (δe) for a system that is giving off 65.0 kj of heat and is performing 855 j of work on the surroundings. 9.00 x 102 kj 64.1 kj -9.00 x 102 kj -64.1 kj -65.9 kj

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 03:00
Schrodinger and heisenberg developed an alternate theory about atomic nature that contradicted some of bohr's model of the atom. how do changes resulting from new technology and evidence affect the reputation of the atomic theory?
Answers: 1

Chemistry, 22.06.2019 04:30
Why are people not able to scuba dive in the deep part of the ocean
Answers: 2

Chemistry, 22.06.2019 09:40
In the lab, ammonia was mixed with water to form ammonium hydroxide. what is/are the reactant(s)? o water and ammonia o ammonia o ammonium hydroxide need
Answers: 2

Chemistry, 22.06.2019 10:00
How many mmols of tris-hcl are there in 100 ml of a 100 mm tris-hcl buffer solution at ph 8.1? note that the 100 mm refers to the sum of tris and tris-hcl concentrations?
Answers: 3
You know the right answer?
Calculate the change internal energy (δe) for a system that is giving off 65.0 kj of heat and is per...
Questions




Mathematics, 29.01.2021 04:00

Mathematics, 29.01.2021 04:00



Advanced Placement (AP), 29.01.2021 04:00



Health, 29.01.2021 04:00

Mathematics, 29.01.2021 04:00

English, 29.01.2021 04:00

Social Studies, 29.01.2021 04:00


Mathematics, 29.01.2021 04:00

Mathematics, 29.01.2021 04:00



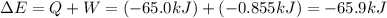
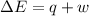
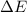 =Change in internal energy
=Change in internal energy
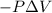 {Work is done by the system is negative as the final volume is greater than initial volume}
{Work is done by the system is negative as the final volume is greater than initial volume}