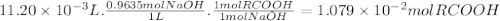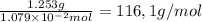Chemistry, 25.11.2019 23:31 golffuture666
What is the molecular weight of a monoprotic carboxylic acid if 11.20 ml of 0.9635 m sodium hydroxide is required to titrate 1.253 g of this acid? the reaction is represented by following equation. note: in the equation, r represents an unspecified carbon containing structure rco-h (aq)naoh (aq) rco, na (aq) hoh (u) separate experiments suggest the unknown acid is likely pentanoic acid, c4h, co2h. is the unknown pentanoic acid? why or why not?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 00:30
Used the balanced equation 2h2+ o2 - -> 2h2o. if you have 7.2 grams of o2 , how many grams of h2o can you produce ?
Answers: 2

Chemistry, 22.06.2019 14:00
Anthracite is so hard and pure it is also referred to as a renewable resource metamorphic rock hot bituminous coal dirty fuel
Answers: 1

Chemistry, 22.06.2019 14:20
7. in the cycle, a virus integrates its dna into the host's dna, and its dna is replicated when the host dna is replicated. a. infectious b. retroviral c. lysogenic d.lytic
Answers: 1

You know the right answer?
What is the molecular weight of a monoprotic carboxylic acid if 11.20 ml of 0.9635 m sodium hydroxid...
Questions



Mathematics, 07.05.2020 03:13


History, 07.05.2020 03:13





Mathematics, 07.05.2020 03:13



Mathematics, 07.05.2020 03:13

Mathematics, 07.05.2020 03:13



History, 07.05.2020 03:13

Mathematics, 07.05.2020 03:13

English, 07.05.2020 03:13

Computers and Technology, 07.05.2020 03:13