Chemistry, 25.11.2019 23:31 ZeroFrost7899
Calculate δh∘f (in kilojoules per mole) for benzene, c6h6, from the following data: 2c6h6(l) + 15o2(g)→12co2(g)+6h2o(l) δh∘=−6534.0 kjδh∘f co2=−393.5 kj/molδh∘f h2o=−285.8 kj/mol express the enthalpy change in kilojoules per mole to three significant digits.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 07:30
Given that 1 mi = 1760 yd, determine what conver- sion factor is appropriate to convert 1849 yd to miles; to convert 2.781 mi to yards.
Answers: 2

Chemistry, 22.06.2019 10:10
Stage in which a typical star has completely stopped fusion
Answers: 1

Chemistry, 22.06.2019 12:40
When 13.3 g koh is dissolved in 102.7 g of water in a coffee-cup calorimeter, the temperature rises from 21.4 °c to 31.53 °c. what is the enthalpy change per gram of koh (j/g) dissolved in the water? * take the density of water as 1.00 g/ml. * assume that the solution has a specific heat capacity of 4.18 j/g*k. enter to 1 decimal place. do not forget the appropriate sign /(+). canvas may auto-delete the (+) sign
Answers: 2

Chemistry, 22.06.2019 16:00
How does blood clotting prevent the entry of pathogens through cuts and wounds? answer asap,, this is due tomorrow. will mark as brainliest or whatever you call it : )
Answers: 2
You know the right answer?
Calculate δh∘f (in kilojoules per mole) for benzene, c6h6, from the following data: 2c6h6(l) + 15o2...
Questions

Mathematics, 18.08.2021 22:30


Computers and Technology, 18.08.2021 22:30





History, 18.08.2021 22:30

Mathematics, 18.08.2021 22:30



Mathematics, 18.08.2021 22:30








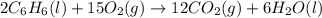
![\Delta H=\sum [n\times \Delta H_f(product)]-\sum [n\times \Delta H_f(reactant)]](/tpl/images/0390/4702/76c37.png)
![\Delta H=[(n_{CO_2}\times \Delta H_{CO_2})+(n_{H_2O}\times \Delta H_{H_2O})]-[(n_{O_2}\times \Delta H_{O_2})+(n_{C_6H_6}\times \Delta H_{C_6H_6})]](/tpl/images/0390/4702/15aa8.png)
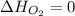 (as heat of formation of substances in their standard state is zero
(as heat of formation of substances in their standard state is zero![-6534.0=[(12\times -393.5)+(6\times -285.8)]-[(15\times 0)+(2\times \Delta H_{C_6H_6})]](/tpl/images/0390/4702/ed6d2.png)