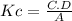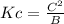Chemistry, 25.11.2019 21:31 FrankyV3220
Write an equilibrium expression for each chemical equation involving one or more solid or liquid reactants or products. part a: hcho2(aq)+h2o(l)⇌h3o+(aq)+cho−2(aq) use a for [hcho2], b for [h2o], c for [h3o+], d for [cho−2].
part b: co2−3(aq)+h2o(l)⇌hco−3(aq)+oh−(aq) use a for [co2−3], b for [h2o], c for [hco−3], d for [oh−].
part c: 2c(s)+o2(g)⇌2co(g) use a for [c], b for [o2], c for [co].
part d: c(s)+co2(g)⇌2co(g) use a for [c], b for [co2], c for [co].

Answers: 3


Another question on Chemistry

Chemistry, 20.06.2019 18:02
Explain the effects of nh3 and hcl on the cuso4 solution in terms of le chatelier's principle
Answers: 2

Chemistry, 22.06.2019 02:30
When you perform this reaction, what could remain at the end of the reaction? check all that apply. excess reactant aqueous copper chloride excess reactant aluminum oxygen product solid copper carbon dioxide product aqueous aluminum chloride water
Answers: 2

Chemistry, 22.06.2019 10:00
How many mmols of tris-hcl are there in 100 ml of a 100 mm tris-hcl buffer solution at ph 8.1? note that the 100 mm refers to the sum of tris and tris-hcl concentrations?
Answers: 3

Chemistry, 22.06.2019 10:50
Someone offer some answers to this, i will give 98 coins and mark as brainliest! i will put the rest of the lab down in the comments,solutions pre-lab questions: in this lab, you will make fruit drinks with powdered drink mix. complete the pre-lab questions to get the values you need for your drink solutions. calculate the molar mass of powered fruit drink mix, made from sucrose (c12h22o11).using stoichiometry, determine the mass of powdered drink mix needed to make a 1.0 m solution of 100 ml. (hint: use molarity = to find the moles of drink mix, then convert moles to grams using a mole conversion.)what mass of powdered drink mix is needed to make a 0.5 m solution of 100 ml?
Answers: 1
You know the right answer?
Write an equilibrium expression for each chemical equation involving one or more solid or liquid rea...
Questions




History, 22.11.2021 17:10

Mathematics, 22.11.2021 17:10


Mathematics, 22.11.2021 17:10

Mathematics, 22.11.2021 17:10


Computers and Technology, 22.11.2021 17:10

Social Studies, 22.11.2021 17:10

Mathematics, 22.11.2021 17:10


Mathematics, 22.11.2021 17:10

Mathematics, 22.11.2021 17:10


Mathematics, 22.11.2021 17:10


Mathematics, 22.11.2021 17:10