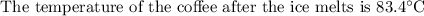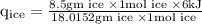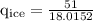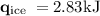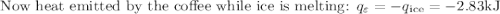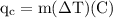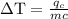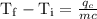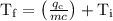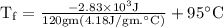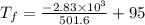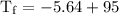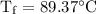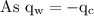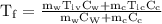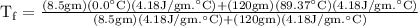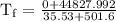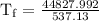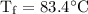Chemistry, 25.11.2019 21:31 jenorajordan5387
An ice cube of mass 8.5g is added to a cup of coffee, whose temperature is 95 degrees celcius and which contains 120 g of liquid. assume the specific heat capacity of coffee is the same as that of water. the heat of fusion of the ice (the heat associated with ice melting) is 6.0 kj/mol. find the temperature of the coffee after the ice melts.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 06:30
The minerals found in bones are deposited by living cells called
Answers: 1

Chemistry, 22.06.2019 09:20
How have the greenhouse gasses increased from the year 2000 to 2018
Answers: 2

Chemistry, 22.06.2019 11:00
3) in peaches, [oh]=3.16x10-11 m a) find [h+ ] b) what is the ph? c) is the solution acidic, basic, or neutral?
Answers: 1

Chemistry, 23.06.2019 06:30
How can the number of core electrons be determined from the periodic table
Answers: 1
You know the right answer?
An ice cube of mass 8.5g is added to a cup of coffee, whose temperature is 95 degrees celcius and wh...
Questions

Chemistry, 29.01.2021 16:50

Business, 29.01.2021 16:50


Mathematics, 29.01.2021 16:50



Mathematics, 29.01.2021 16:50

Mathematics, 29.01.2021 16:50



Computers and Technology, 29.01.2021 16:50

Mathematics, 29.01.2021 16:50





Computers and Technology, 29.01.2021 16:50