
Chemistry, 25.11.2019 20:31 jakobrobinette
Kemmi major does some experimental work on the combustion of sucrose: c12h22o11(s) 12 o2(g) → 12 co2(g) 11 h2o(g) she burns a 0.05392 g pellet of sucrose in a bomb calorimeter with excess oxygen. she determines the qrxn to be –916.6 j for the reaction. calculate the ∆h value for the combustion reaction. (round the answer to 3 significant digits, units of kj, pay attention to positive or negative.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 04:00
Actual ingredients of lab (the cookies i am actually making) 1/2 cup sugar 1/2 cup brown sugar 1 1/3 stick margarine 1 egg 1/2 tsp salt 1 tsp vanilla 1/2 tsp baking soda 1 1/2 cup flour 1 1/3 cup chocolate chip can you answer the questions below ? discussion 1. suppose you are given the following amounts of ingredients: 1 dozen eggs 24 tsp. of vanilla 1 lb. (82 tsp.) of salt 1 lb. (84 tsp.) of baking soda 3 cups of chocolate chips 5 lb. (11 cups) of sugar 2 lb. (4 cups) of brown sugar 1 lb. (4 sticks) of margarine a. for each ingredient, calculate how many cookies could be prepared if all of that ingredient were consumed. (for example, the recipe shows that using 1 egg- with the right amounts of the other ingredients- yields 24 cookies. how many cookies can you make if the recipe is increased proportionately for 12 eggs? ) b. to determine the limiting reactant for the new ingredients list, identify which ingredient will result in the fewest number of cookies. c. what is the maximum number of cookies that can be produced from the new amounts of ingredients?
Answers: 1

Chemistry, 22.06.2019 08:00
Why is the bond angle in a water molecule less than the bond angle of methane? a. the central oxygen atom in water has two lone pairs of electrons, whereas the central carbon atom in methane has no lone pairs. b. the central hydrogen atom in water has one lone pair of electrons, whereas the central carbon atom in methane has two lone pairs. c. the central oxygen atom in water has four lone pairs of electrons, whereas the central carbon atom in methane has only one lone pair. d. the central oxygen atom exerts more repulsive force on surrounding atoms than the central carbon atom in methane does. reset next
Answers: 2

Chemistry, 22.06.2019 16:00
Click the button that shows the correct relationship of the electron affinities of the elements sodium and phosphorus. sodium’s electron affinity value is more negative than the electron affinity value of phosphorus. phosphorus’ electron affinity value is more negative than the electron affinity value of sodium. this information cannot be determined using the periodic table. answer is b on e2020.
Answers: 3

Chemistry, 23.06.2019 01:00
Which substance—wood or silver—is the better thermal conductor? a thermal conductor is a material that requires very little heat energy to change its temperature. explain your answer.
Answers: 3
You know the right answer?
Kemmi major does some experimental work on the combustion of sucrose: c12h22o11(s) 12 o2(g) → 12 co...
Questions

English, 23.10.2020 16:40




Mathematics, 23.10.2020 16:40



Chemistry, 23.10.2020 16:40

Mathematics, 23.10.2020 16:40

History, 23.10.2020 16:40


Physics, 23.10.2020 16:40


Biology, 23.10.2020 16:40

Mathematics, 23.10.2020 16:40

Biology, 23.10.2020 16:40



Mathematics, 23.10.2020 16:40

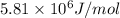
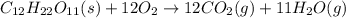
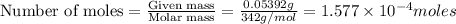
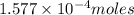 of sucrose releases = 916.6 J of heat
of sucrose releases = 916.6 J of heat
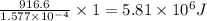 of heat
of heat


