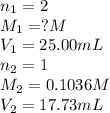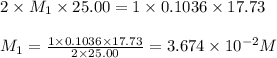Chemistry, 25.11.2019 19:31 michellectucker1982
Astudent titrated a 25.00-ml sample of a solution containing an unknown weak, diprotic acid (h2a) with n2oh. if the titration required 17.73 ml of 0.1036 m n2oh to completely neutralize the acid, calculate the concentration (in m) of the weak acid in the sample.
(a) 9.184 x 10 m
(b) 3.674 x 10-2 m
(c) 7.304 x 10-2 m
(d) 7.347 x 10-2 m
(e) 1.469 x 101 m

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 00:10
Think about how you can use le chatelier’s principle to find possible solutions to the design problem. describe at least two ways to increase the yield (amount) of ammonia based on this principle.
Answers: 2

Chemistry, 22.06.2019 03:30
Asample of ammonia reacts with oxygen as shown. 4nh3(g) + 5o2(g) 4no(g) + 6h2o(g) what is the limiting reactant if 4.0 g of nh3 react with 8.0 g of oxygen? o2 because it produces only 0.20 mol of no. nh3 because it produces only 0.20 mol of no. o2 because it produces two times less no than nh3. nh3 because it produces three times more no than o2.
Answers: 3

Chemistry, 22.06.2019 12:00
What are the first two quantum numbers for the electrons located in subshell 4d? what are the first three quantum numbers for the electrons located in subshell 2s? how many electrons can be held in a sublevel l = 3? how many electrons can be held in the energy level n = 4? how many electrons in an atom can share the quantum numbers n = 4 and l = 3?
Answers: 1

Chemistry, 22.06.2019 14:50
The table compares the number of electrons in two unknown neutral atoms. comparison of electrons atom number of electrons a 9 d 11 use this information to determine the number of valence electrons in the atoms. which of the following correctly compares the stability of tthe table compares the number of electrons in two unknown neutral atoms. comparison of electrons atom number of electrons a 9 d 11 use this information to determine the number of valence electrons in the atoms. which of the following correctly compares the stability of the two atoms? both are unreactive. both are highly reactive. a is unreactive and d is reactive. a is reactive and d is unreactive.
Answers: 3
You know the right answer?
Astudent titrated a 25.00-ml sample of a solution containing an unknown weak, diprotic acid (h2a) wi...
Questions


Mathematics, 09.09.2021 21:20



Mathematics, 09.09.2021 21:20

Biology, 09.09.2021 21:20

Chemistry, 09.09.2021 21:20


Mathematics, 09.09.2021 21:20

History, 09.09.2021 21:20

Business, 09.09.2021 21:20



Mathematics, 09.09.2021 21:20

Advanced Placement (AP), 09.09.2021 21:20


Chemistry, 09.09.2021 21:20

Biology, 09.09.2021 21:20


History, 09.09.2021 21:20

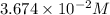
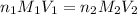
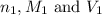 are the n-factor, molarity and volume of acid which is
are the n-factor, molarity and volume of acid which is 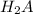
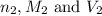 are the n-factor, molarity and volume of base which is NaOH.
are the n-factor, molarity and volume of base which is NaOH.