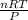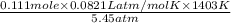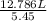Chemistry, 17.12.2019 04:31 khikhi1705
What would be the volume in liters of an ideal gas, if a 0.111 mole sample of the gas had a temperature of 1130 degrees celsius at a pressure of 5.45 atmospheres? (the ideal gas constant is 0.0821 l•atm/mol•k.)
0.43 liters
1.43 liters
1.89 liters
2.35 liters

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 10:30
What determines the average kinetic energy of the particles in a gas? a. the number of collisions b. the number of particles c. the size of the particles d. the temperature
Answers: 1

Chemistry, 22.06.2019 22:00
Plz ill give u brainliest which of the following steps is not likely to take place during cellular respiration? a.oxygen combines with carbon of simple sugar. b. energy molecule transfers energy to cells. d. energy is used up.
Answers: 3

Chemistry, 23.06.2019 02:30
Which statement best describes the liquid state of matter? a. it has definite shape but indefinite volume. b. it has definite shape and definite volume. c. it has indefinite shape and indefinite volume. d. it has indefinite shape but definite volume.
Answers: 1
You know the right answer?
What would be the volume in liters of an ideal gas, if a 0.111 mole sample of the gas had a temperat...
Questions

Mathematics, 02.12.2020 04:10

History, 02.12.2020 04:10

Mathematics, 02.12.2020 04:10

English, 02.12.2020 04:10

Mathematics, 02.12.2020 04:10

French, 02.12.2020 04:10


Mathematics, 02.12.2020 04:10



Mathematics, 02.12.2020 04:10


Mathematics, 02.12.2020 04:10

Health, 02.12.2020 04:10



Mathematics, 02.12.2020 04:10

English, 02.12.2020 04:10


Mathematics, 02.12.2020 04:10