Chemistry, 23.11.2019 07:31 langel7373
A23.0 g piece of metal at 99.0 ∘c is placed in a calorimeter containing 53.2 g of water at 24.0 ∘c. the final temperature of the mixture is 26.1 ∘c. what is the specific heat capacity of the metal? assume no energy is lost to the surroundings.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 22:00
During chemistry class, carl performed several lab tests on two white solids. the results of three tests are seen in the data table. based on this data, carl has concluded that substance b must have bonds.
Answers: 2

Chemistry, 22.06.2019 01:40
Brainliesttt me asap! the below reaction can be categorized as more than one type of reaction. which reactions are these, and what are the types of reactions?
Answers: 1

Chemistry, 22.06.2019 08:30
Which of the following would be an accurate picture of the earth during the summer time of the northern hemisphere?
Answers: 1

Chemistry, 22.06.2019 18:00
How many moles of oxygen gas are produced from the decomposition of six moles of potassium chlorate
Answers: 3
You know the right answer?
A23.0 g piece of metal at 99.0 ∘c is placed in a calorimeter containing 53.2 g of water at 24.0 ∘c....
Questions



History, 19.05.2020 22:03



Computers and Technology, 19.05.2020 22:03



World Languages, 19.05.2020 22:03








Biology, 19.05.2020 22:03



Biology, 19.05.2020 22:03

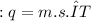
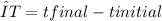 , m the mass and s the specific heat.
, m the mass and s the specific heat.
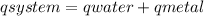
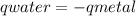
![mw.sw.ΔTw = -[mm.sm.ΔTm ]](/tpl/images/0387/7653/567ae.png)
![53.2 g . 4.184 J/g°C . (26.1 - 24.0)ºC = -[23.0 g . sm . (26.1 - 99.0)°C]](/tpl/images/0387/7653/a0fbc.png)
![464.436 J = -[23.0 g . sm . (-72.9)°C]](/tpl/images/0387/7653/3ce49.png)
![sm = 464.436 J/ -[-1676.7 g°C]](/tpl/images/0387/7653/30209.png)