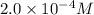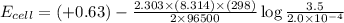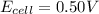Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 07:10
Remember to use the proper number of significant figures and leading zeros in all calculations.gelatin has a density of 1.27 g/cm³. if you have a blob of gelatin dessert that fills a 2.0 liter bottle, what is its mass? 2540 g2500 g3.9 x 10-43.937x 10-4
Answers: 3

Chemistry, 22.06.2019 07:30
In a reaction (at equilibrium) that makes more moles of gas than it consumes, what is the effect of increasing the pressure?
Answers: 1

Chemistry, 22.06.2019 22:30
What methods could you use to solubilize calcium carbonate
Answers: 1

Chemistry, 23.06.2019 01:00
If i had 2 m naoh solution, what does the 2 m stand for? 2 molar, but 2 of a solute in 1
Answers: 1
You know the right answer?
The standard cell potential (e°cell) for the reaction below is +0.63 v. the cell potential for this...
Questions









Physics, 16.04.2020 19:11


Computers and Technology, 16.04.2020 19:11



Mathematics, 16.04.2020 19:11





Mathematics, 16.04.2020 19:11

Mathematics, 16.04.2020 19:11

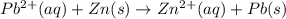
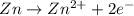
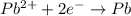
![E_{cell}=E^o_{cell}-\frac{2.303RT}{nF}\log \frac{[Zn^{2+}]}{[Pb^{2+}]}](/tpl/images/0387/6329/1472e.png)
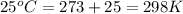
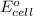 = standard electrode potential of the cell = +0.63 V
= standard electrode potential of the cell = +0.63 V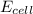 = cell potential for the reaction = ?
= cell potential for the reaction = ?![[Zn^{2+}]](/tpl/images/0387/6329/9c01a.png) = 3.5 M
= 3.5 M![[Pb^{2+}]](/tpl/images/0387/6329/0acfd.png) =
=