
Chemistry, 23.11.2019 04:31 Jenifermorales101
It turns out that the van der waals constant b equals four times the total volume actually occupied by the molecules of a mole of gas.
using this figure, calculate the fraction of the volume in a container actually occupied by ar atoms at 230 atm pressure and 0 c. assume b=0.0322 l/mol

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 16:30
How many atoms of oxygen are contained in 160 grams of n2o3
Answers: 2

Chemistry, 22.06.2019 05:00
What forms when chemical reactions combine pollution with sunlight?
Answers: 1

Chemistry, 22.06.2019 12:00
What is the lowest number energy level where a d sublevel is found
Answers: 1

You know the right answer?
It turns out that the van der waals constant b equals four times the total volume actually occupied...
Questions


Mathematics, 07.05.2021 15:20

History, 07.05.2021 15:20

English, 07.05.2021 15:20


Mathematics, 07.05.2021 15:20


English, 07.05.2021 15:20

Social Studies, 07.05.2021 15:20

History, 07.05.2021 15:20


Mathematics, 07.05.2021 15:20


World Languages, 07.05.2021 15:20

Mathematics, 07.05.2021 15:20

History, 07.05.2021 15:20


Mathematics, 07.05.2021 15:20

Social Studies, 07.05.2021 15:20

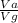
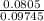 = 0.0826
= 0.0826

