50 points and will mark !
1. mary bought an apple for lunch. she found the weight of the appl...

Chemistry, 23.11.2019 04:31 zakarycrane8101
50 points and will mark !
1. mary bought an apple for lunch. she found the weight of the apple on the spring scale in the store and estimated how much her apple would cost. when she got home she washed the apple, cut it in half, and placed it in a zip-lock bag. mary refrigerated the apple over night. the next morning the apple, was crisp, cold, and the cut surfaces had turned brown.
mary cut the apple in half is an example of
a) sublimation.
b) a chemical change.
c) a physical change.
d) a change of state.
2. which statement best describes the nuclear model of the atom?
a) negative charges dispersed in a positively charged cloud
b) positive charges dispersed in a negatively charged cloud
c) small, dense positive nucleus surrounded by a diffuse negatively charged cloud
d) small, dense negative nucleus surrounded by a diffuse positively charged cloud
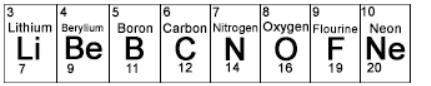
3. which of these elements would have the largest atomic radius? (picture at the bottom)
a) c
b) f
c) li
d) ne

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 09:30
Apump contains 0.5 l of air at 203 kpa.you draw back on the piston of the pump, expanding the volume until the pressure reads 50.8 kpa. what is the new volume of the air pump
Answers: 2

Chemistry, 23.06.2019 04:40
Temperature is defined as a. the equivalent of heat. b. a measure of the average kinetic energy of the individual atoms or molecules composing a substance. c. how hot or cold it is. d. the total kinetic energy of the atoms or molecules composing a substance. e. none of the above is correct.
Answers: 1

Chemistry, 23.06.2019 07:00
Under what conditions will a gas be most likely to exhibit the ideal gas properties predicted by the ideal gas law? 1)high pressures and high temperature, because particles are forced closer together with higher kinetic energy, so intermolecular forces of attraction are weaker 2)high pressure and low temperature, because particles are forced closer together and moving slower, so the volume of the particles is less significant 3) low pressure and high temperature, because particles are spread farther apart and moving faster, so the intermolecular forces of attraction are weaker 4)low pressure and low temperature, because particles are spread farther apart with lower kinetic energy, so the volume of the particles is less significant
Answers: 2

Chemistry, 23.06.2019 08:00
Suppose a pair of chemical compounds a and b can react in two different ways: a + b -> c reaction 1 gives product c. a + b -> d reaction 2 gives product d. the following facts are known about the two reactions: . reaction 1 is endothermic and reaction 2 is exothermic. if a reaction vessel is charged (filled) with a and b , then at first d is produced faster than c. use these facts to sketch a qualitative reaction energy diagram for both reactions. note: because these sketches are only qualitative, the energies don? t have to be exact. they only have to have the right relationship to each other. for example, if one energy is less than another, that fact should be clear in your sketch.
Answers: 3
You know the right answer?
Questions

Biology, 03.11.2020 04:20

Mathematics, 03.11.2020 04:20

Mathematics, 03.11.2020 04:20


Mathematics, 03.11.2020 04:20





Mathematics, 03.11.2020 04:20

Mathematics, 03.11.2020 04:20


Mathematics, 03.11.2020 04:20


English, 03.11.2020 04:20

Social Studies, 03.11.2020 04:20

Mathematics, 03.11.2020 04:20



Mathematics, 03.11.2020 04:20



