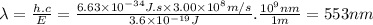The lines on absorption atomic spectra correspond to energies needed for electrons to be excited from a lower energy level to a higher energy level. assume that the energy needed for an electron in 2p orbital in an o atom to jump to 3s orbital is 3.6*10^-19 j, what is its wavelength of the line atomic spectra in nanometer (nm)?
note: use whole numbers and 3 sig figs, or no decimal place.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 08:00
An electron moved from shell n = 2 to shell n = 1. what most likely happened during the transition? a fraction of a photon was added. a photon of energy was absorbed. a fraction of a photon was removed. a photon of energy was released.
Answers: 1


Chemistry, 22.06.2019 19:30
What is the common name for the compound shown here? enter the common name of the compound shown?
Answers: 2

Chemistry, 22.06.2019 19:50
Identify the lewis base in this balanced equation: fe3+ h2o fe(h2o)63+
Answers: 1
You know the right answer?
The lines on absorption atomic spectra correspond to energies needed for electrons to be excited fro...
Questions

Mathematics, 07.05.2020 05:12


History, 07.05.2020 05:12


Mathematics, 07.05.2020 05:12

Mathematics, 07.05.2020 05:12




History, 07.05.2020 05:12

Social Studies, 07.05.2020 05:12

Geography, 07.05.2020 05:12



Computers and Technology, 07.05.2020 05:12



Mathematics, 07.05.2020 05:12