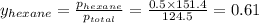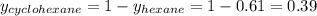Determine the mole fractions of each component in the vapor phase of the vapor in equilibrium with a 1: 1 molar ratio of hexane (c6h14) and cyclohexane (c6h12). the equilibrium vapor pressures of hexane and cyclohexane are equal to 151.4 torr and 97.6 torr respectively

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 09:40
Apiece of copper has a temperature of 75.6 0c. when the metal is placed in 100.0 grams of water at 19.1 0c, the temperature rises by 5.5 0c. what is the mass of the metal?
Answers: 1

Chemistry, 22.06.2019 23:30
The ammonia molecule in the diagram has the observed bond orientation because
Answers: 1

Chemistry, 23.06.2019 03:00
The size (radius) of an oxygen molecule is about 2.0 ×10−10m. make a rough estimate of the pressure at which the finite volume of the molecules should cause noticeable deviations from ideal-gas behavior at ordinary temperatures (t= 300k ). assume that deviatons would be noticeable when volume of the gas per molecule equals the volume of the molecule itself.
Answers: 3

You know the right answer?
Determine the mole fractions of each component in the vapor phase of the vapor in equilibrium with a...
Questions

Mathematics, 25.06.2019 10:30

Social Studies, 25.06.2019 10:30








Social Studies, 25.06.2019 10:30

Mathematics, 25.06.2019 10:30




Social Studies, 25.06.2019 10:30

History, 25.06.2019 10:30

Mathematics, 25.06.2019 10:30

History, 25.06.2019 10:30


Spanish, 25.06.2019 10:30

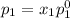 and
and 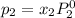
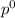 = pressure in the pure state
= pressure in the pure state
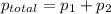
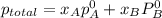
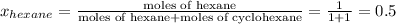 ,
, 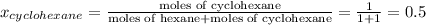 ,
, 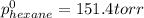
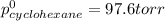
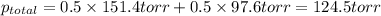
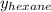 = mole fraction of hexane in vapor phase
= mole fraction of hexane in vapor phase