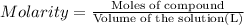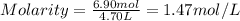Chemistry, 22.11.2019 23:31 shealwaysknows23
An aqueous magnesium chloride solution is made by dissolving 6.90 moles of mgcl 2 in sufficient water so that the final volume of the solution is 4.70 l . calculate the molarity of the mgcl 2 solution.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 02:10
26. of of (aq) by (aq) is . if 50.00 ml of 1.05 m is to 25.00 ml of 1.86 m ,at be? ( no is toina of aof) , h.. (p. ). . .
Answers: 3

Chemistry, 22.06.2019 05:30
What happens to the atomic radius when an elctron is lost
Answers: 1

Chemistry, 22.06.2019 14:00
Anthracite is so hard and pure it is also referred to as a renewable resource metamorphic rock hot bituminous coal dirty fuel
Answers: 1

Chemistry, 23.06.2019 06:30
Consider the heating curve of h2o and line segments a, b, and c. several changes are taking place at a, b, and c. all but one would be an appropriate description as e move through segments a, b and then c.
Answers: 3
You know the right answer?
An aqueous magnesium chloride solution is made by dissolving 6.90 moles of mgcl 2 in sufficient wate...
Questions

Mathematics, 05.02.2020 10:00

English, 05.02.2020 10:00






History, 05.02.2020 10:00




Biology, 05.02.2020 10:00



English, 05.02.2020 10:00