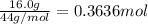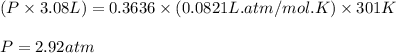Chemistry, 22.11.2019 23:31 oliviajewelwilliams
Cyclists sometimes use pressurized carbon dioxide inflators to inflate a bicycle tire in the event of a flat. these inflators use metal cartridges that contain 16.0 g of carbon dioxide.
at 301 k , to what pressure (in psi) can the carbon dioxide in the cartridge inflate a 3.08 l mountain bike tire?
(note: the gauge pressure is the difference between the total pressure and atmospheric pressure. in this case, assume that atmospheric pressure is 14.7 psi.)
express your answer to three significant figures with the appropriate units.

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 10:30
What determines the average kinetic energy of the particles in a gas? a. the number of collisions b. the number of particles c. the size of the particles d. the temperature
Answers: 1


Chemistry, 22.06.2019 17:50
You exhale co2 which is produced during cellular respiration. co2 combines with the water in your blood's plasma to make up one half of the body's most important buffer pair, carbonic acid. the more physical activity you engage in, the more co2 your body is producing. you can see this by putting some of the cabbage indicator in a glass and then blowing bubbles into it through a straw. can you see a change in the color of the indicator?
Answers: 2
You know the right answer?
Cyclists sometimes use pressurized carbon dioxide inflators to inflate a bicycle tire in the event o...
Questions

Mathematics, 22.10.2019 04:00


Arts, 22.10.2019 04:00

Mathematics, 22.10.2019 04:00

Biology, 22.10.2019 04:00


SAT, 22.10.2019 04:00


Mathematics, 22.10.2019 04:00







Chemistry, 22.10.2019 04:00


Geography, 22.10.2019 04:00


History, 22.10.2019 04:00