
Chemistry, 22.11.2019 21:31 samueltaye
Calculate δh and δstot when two copper blocks, each of mass 10.0 kg, one at 100°c and the other at 0°c, are placed in contact in an isolated container. the specific heat capacity of copper is 0.385 j k−1 g−1 and may be assumed constant over the temperature range involved.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:00
Afamily is one another name for a group on the table of elements.
Answers: 1

Chemistry, 22.06.2019 00:00
The pressure inside a hydrogen-filled container was 2.10 atm at 21 ? c. what would the pressure be if the container was heated to 92 ? c ?
Answers: 2

Chemistry, 22.06.2019 02:40
Achange in the number of neutrons in an atom will change an blank . when the number of protons changes in an atom, a new element will form.
Answers: 2

Chemistry, 22.06.2019 08:00
This classification of drug typically changes the brain's chemistry and reduces its ability to create its own endorphins.
Answers: 1
You know the right answer?
Calculate δh and δstot when two copper blocks, each of mass 10.0 kg, one at 100°c and the other at 0...
Questions

Mathematics, 24.06.2020 22:01

Mathematics, 24.06.2020 22:01




Mathematics, 24.06.2020 22:01

Mathematics, 24.06.2020 22:01




World Languages, 24.06.2020 23:01

Mathematics, 24.06.2020 23:01

Mathematics, 24.06.2020 23:01

Mathematics, 24.06.2020 23:01

Mathematics, 24.06.2020 23:01


English, 24.06.2020 23:01



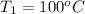 = (100 + 273) K = 373 K
= (100 + 273) K = 373 K 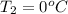 = (0 + 273) K = 273 K
= (0 + 273) K = 273 K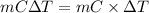
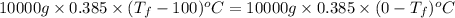
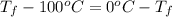
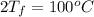
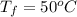
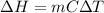
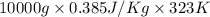
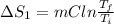
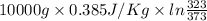
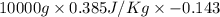
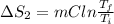
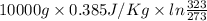
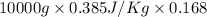
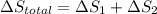
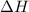 is 1243.5 kJ and
is 1243.5 kJ and 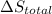 is 93.37 J/K.
is 93.37 J/K.

