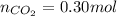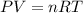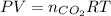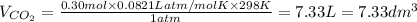Chemistry, 04.11.2019 14:31 danielzgame
In an experiment, 12.0 dm3 of oxygen, measured under room conditions, is used to burn completely 0.10 mol of propan-1-ol. what is the final volume of gas, measured under room conditions? a 7.20 dm3 b 8.40 dm3 c 16.8 dm3 d 18.00 dm3

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 18:30
For each of the following mixtures decide if filtering would be suitable to separate the substances. explain your answers. oil in water sugar in water sand in water chalk in water tea leaves in a cup of tea
Answers: 2

Chemistry, 22.06.2019 09:30
The chart shows the bid provided by four contractors to complete a job. which contractor is the most cost-effective?
Answers: 3

Chemistry, 22.06.2019 10:30
Aglow stick contains a glass vial with chemicals. when the glow stick is bent, the vial breaks and the chemicals react to produce a glow. a science student observes that a glow stick kept in the freezer glows for a longer duration than a glow stick kept at room temperature. what conclusion can be drawn based on the observation? be sure to note the outcome and test variables in the conclusion.
Answers: 1

Chemistry, 22.06.2019 16:00
About 3% of the water on earth is freshest. only about 40% of that freshwater is available for human use. why is so much freshwater unavailable for human use?
Answers: 2
You know the right answer?
In an experiment, 12.0 dm3 of oxygen, measured under room conditions, is used to burn completely 0.1...
Questions

Mathematics, 16.02.2021 20:00

Mathematics, 16.02.2021 20:00

Mathematics, 16.02.2021 20:00

Mathematics, 16.02.2021 20:00


English, 16.02.2021 20:00



Mathematics, 16.02.2021 20:00

Mathematics, 16.02.2021 20:00

Mathematics, 16.02.2021 20:00

Biology, 16.02.2021 20:00


Mathematics, 16.02.2021 20:00

Mathematics, 16.02.2021 20:00

English, 16.02.2021 20:00

Mathematics, 16.02.2021 20:00



Mathematics, 16.02.2021 20:00

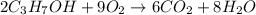
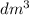 )
)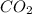 then, 0.10 moles of propanol will give:
then, 0.10 moles of propanol will give: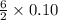 moles of
moles of